爿片刀客
· 湖南
$Biomea Fusion(BMEA)$ Biomea Fusion (BMEA.US)
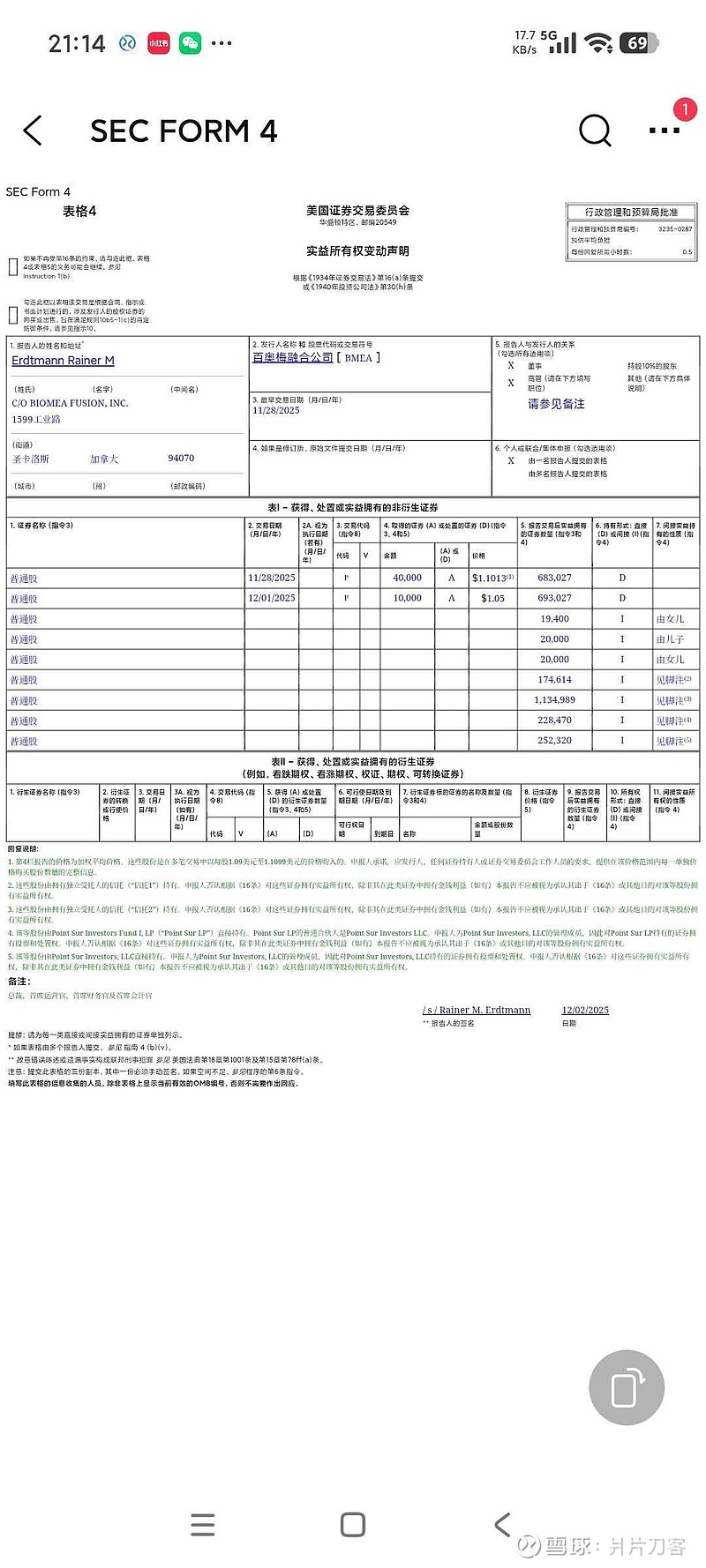
1美元以下的货都被高管买走了。那天,我犹豫了好久,在9毛的时候。我在犹豫什么?我在徘徊什么?![[哭泣] [哭泣]](//assets.imedao.com/ugc/images/face_regular/v1/emoji_09_cry.png?v=1)
![[哭泣] [哭泣]](//assets.imedao.com/ugc/images/face_regular/v1/emoji_09_cry.png?v=1)
![[哭泣] [哭泣]](//assets.imedao.com/ugc/images/face_regular/v1/emoji_09_cry.png?v=1) 9毛的时候真的是吓怕了,但确实那时候才是买入的最佳时机。会不会像前面那$Capricor疗法(CAPR)$ 一样,一个晚上给干6倍!
9毛的时候真的是吓怕了,但确实那时候才是买入的最佳时机。会不会像前面那$Capricor疗法(CAPR)$ 一样,一个晚上给干6倍!
Biomea Fusion Presents COVALENT-111 Study Results at the 23rd World Congress on Insulin Resistance, Diabetes & Cardiovascular Disease (WCIRDC)
Biomea Fusion在第23届世界胰岛素抵抗、糖尿病与心血管疾病大会(WCIRDC)上展示了COVALENT-111研究的结果。GlobeNewswire· 20:00
8分钟
Biomea Fusion(BMEA.US)
1.1901.330
+13.33%
+11.76%
Durable Glycemic and C-Peptide Improvements with Icovamenib in Insulin-Deficient Type 2 Diabetes
在胰岛素缺乏型2型糖尿病中,Icovamenib显示出持久的血糖和C肽改善效果
SAN CARLOS, Calif., Dec. 05, 2025 (GLOBE NEWSWIRE) -- Biomea Fusion, Inc. ("Biomea" or "Biomea Fusion" or "the Company") (Nasdaq: BMEA), a clinical-stage diabetes and obesity company, today announced that it presented COVALENT-111 study results at the 23rd WCIRDC which took place December 3-6, 2025 in Los Angeles, California. The data showed durable glycemic and c-peptide improvements with icovamenib, a menin inhibitor targeting beta-cell restoration in insulin deficient type 2 diabetes.
加利福尼亚州圣卡洛斯,2025年12月5日(GLOBE NEWSWIRE)—— Biomea Fusion, Inc.(“Biomea”或“Biomea Fusion”或“公司”)(纳斯达克:BMEA),一家处于临床阶段的糖尿病和肥胖症公司,今天宣布在2025年12月3日至6日在加利福尼亚州洛杉矶举行的第23届WCIRDC会议上展示了COVALENT-111研究的结果。数据显示,Icovamenib作为一种针对胰岛素缺乏型2型糖尿病β细胞恢复的menin抑制剂,能够带来持久的血糖和C肽改善效果。
"I am very pleased that our data was selected for an oral presentation at the WCIRDC meeting this year. Over the past 24 months, our clinical studies have shown that selective inhibition of menin can meaningfully influence the clinical response of patients with insulin deficient diabetes. The results we presented at this meeting highlight the lasting and continuous benefits observed in in our study, with durable glycemic and C-peptide improvements 9 months after the last dose," said Mick Hitchcock, Ph.D., Interim CEO and Board Member of Biomea Fusion. "Targeting menin offers promise for people living with diabetes, with the potential to support the natural insulin producing capacity of the pancreatic beta cells. Icovamenib, as a selective menin inhibitor, represents a first in class approach in this area of research."
“我很高兴我们的数据被选为今年WCIRDC会议的口头报告。在过去的24个月里,我们的临床研究表明选择性抑制menin可以显著影响胰岛素缺乏型糖尿病患者的临床反应。我们在此次会议上展示的研究结果突显了长期且持续的益处,在最后一剂用药9个月后仍观察到持久的血糖和C肽改善效果,” Biomea Fusion临时首席执行官兼董事会成员Mick Hitchcock博士表示。“靶向menin为糖尿病患者带来了希望,有可能支持胰腺β细胞的天然胰岛素生产能力。作为一种选择性的menin抑制剂,Icovamenib代表了这一研究领域的首创方法。”
Key Highlights from WCIRDC showing HbA1c and C-peptide responses at week 52 – 9 months post the last dose:
WCIRDC的关键亮点显示HbA1c和C肽反应在第52周——最后一剂用药后的9个月:
Icovamenib demonstrated durable and continuous treatment effect in severe insulin-deficient type 2 diabetes (T2D)Higher HbA1c reduction was associated with higher icovamenib exposureIcovamenib improved long-term insulin secretion in severe insulin-deficient T2DTreatment effect in GLP-1 "failures" continued to improve with durable and clinically significant improvements in HbA1cIcovamenib was generally well-tolerated, with no adverse-event related discontinuations and no related serious adverse events
Icovamenib在严重胰岛素缺乏型2型糖尿病(T2D)中表现出持久且连续的治疗效果较高的HbA1c降低幅度与较高的Icovamenib暴露水平相关Icovamenib改善了严重胰岛素缺乏型2型糖尿病的长期胰岛素分泌在GLP-1“失败”患者中的治疗效果继续改善,HbA1c指标持续且具有临床意义的显著改善。Icovamenib总体耐受性良好,未发生与不良事件相关的停药,也无相关严重不良事件。
The abstract will be published in the peer-reviewed Metabolism: Experimental and Clinical. The presentation will be available on Biomea Fusion's Investor Relations Page under the Events. Please find a link here to our website where the poster will be available.
摘要将在同行评议期刊《代谢:实验与临床》上发表。演示文稿将发布在Biomea Fusion的投资者关系页面的“活动”栏目下。请点击此处链接访问我们的网站查看海报。
COVALENT-111 Study Design
COVALENT-111 is a double-blind, randomized, placebo-controlled trial that enrolled adult patients diagnosed with T2D within the last 7 years. Eligible participants had HbA1c levels between 7.0% and 10.5%, and a body mass index (BMI) between 25 and 40 kg/m2. At baseline, all participants were treated with lifestyle management, including diet and exercise, with or without antidiabetic medications and had inadequate glycemic control despite treatment with up to three antidiabetic medications. The study evaluated icovamenib in three dosing regimens: Arm A at 100mg QD (once daily) for 8 weeks, Arm B at 100mg QD for 12 weeks, and Arm C at 100 mg QD for 8 weeks and 100mg BID (twice daily) for 4 weeks.
COVALENT-111 研究设计
COVALENT-111是一项双盲、随机、安慰剂对照试验,纳入了过去7年内确诊为2型糖尿病(T2D)的成年患者。符合条件的参与者HbA1c水平在7.0%至10.5%之间,身体质量指数(BMI)在25至40 kg/m²之间。基线时,所有参与者均接受生活方式管理(包括饮食和运动),无论是否使用抗糖尿病药物,并且尽管接受了多达三种抗糖尿病药物治疗,其血糖控制仍不理想。研究评估了icovamenib的三种剂量方案:A组100mg QD(每日一次)持续8周,B组100mg QD持续12周,C组100mg QD持续8周后改为100mg BID(每日两次)持续4周。
About Icovamenib
Icovamenib is an investigational, orally bioavailable, potent, and selective covalent inhibitor of menin. The proposed mechanism of action for icovamenib in diabetes is selective and partial inhibition of menin, a regulator of beta cell quantity and function, thereby enabling the proliferation, preservation, and reactivation of a patient's own healthy, functional, insulin-producing beta cells. As the first non-chronic therapy for T2D, icovamenib could become an important addition to the diabetes treatment landscape once it has successfully completed its ongoing clinical studies.
About Biomea Fusion
Biomea Fusion is a clinical-stage biopharmaceutical company advancing oral small molecule therapies, icovamenib and BMF-650, for diabetes and obesity. These programs target metabolic disorders, a global health challenge affecting nearly half of Americans and one-fifth of the world's population. Biomea's mission is to deliver transformative treatments that restore health for patients living with diabetes, obesity, and related conditions. We aim to cure.
关于Icovamenib
Icovamenib是一种研究性、口服生物可利用、高效且选择性的共价menin抑制剂。Icovamenib在糖尿病中的作用机制是选择性和部分抑制menin(一种β细胞数量和功能的调节因子),从而促进患者自身健康、功能性胰岛素生成β细胞的增殖、保存和重新激活。作为首个非慢性治疗T2D的疗法,一旦完成正在进行的临床研究,icovamenib有望成为糖尿病治疗领域的重要补充。
关于Biomea Fusion
Biomea Fusion是一家临床阶段的生物制药公司,正在推进口服小分子疗法icovamenib和BMF-650用于治疗糖尿病和肥胖症。这些项目针对代谢紊乱,这是影响近一半美国人和全球五分之一人口的全球性健康挑战。Biomea的使命是为糖尿病、肥胖症及相关疾病的患者提供恢复健康的变革性疗法。我们的目标是治愈。
Visit us at and follow us on LinkedIn, X and Facebook.
请访问我们 并关注我们 领英, X 和 脸书.
Forward-Looking Statements
Statements we make in this press release may include statements which are not historical facts and are considered forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the "Securities Act"), and Section 21E of the Securities Exchange Act of 1934, as amended (the "Exchange Act"). These statements may be identified by words such as "aims," "anticipates," "believes," "could," "estimates," "expects," "forecasts," "goal," "intends," "may," "plans," "possible," "potential," "seeks," "will," and variations of these words or similar expressions that are intended to identify forward-looking statements. Any such statements in this press release that are not statements of historical fact, including statements regarding the clinical and therapeutic potential of our product candidates and development programs, including icovamenib and the potential of icovamenib as a treatment for T1D and T2D, and our expectations regarding the optimal dose and target patient population; our research, development and regulatory plans; the mechanism of action of our product candidates and development programs; the progress and initiation of our ongoing and upcoming clinical trials, including our Food Effect Study (COVALENT-121), the initiation of our Phase IIb trial (COVALENT-211) in severe insulin-deficient type 2 diabetes patients, to initiate in fourth quarter of 2025 and the initiation of our Phase II trial with GLP-1 therapy (COVALENT-212) in type 2 diabetes patients, in the fourth quarter of 2025; the anticipated availability of data from our clinical trials; our planned interactions with regulators, and the timing of such events may be deemed to be forward-looking statements. We intend these forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act and Section 21E of the Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. Any forward-looking statements in this press release are based on our current expectations, estimates and projections only as of the date of this release and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements, including the risk that preliminary or interim results of preclinical studies or clinical trials may not be predictive of future or final results in connection with future clinical trials and the risk that we may encounter delays in preclinical or clinical development, patient enrollment and in the initiation, conduct and completion of our ongoing and planned clinical trials and other research and development activities. These risks concerning Biomea Fusion's business and operations are described in additional detail in its periodic filings with the U.S. Securities and Exchange Commission ("SEC"), including its most recent periodic report filed with the SEC and subsequent filings thereafter. Biomea Fusion explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
前瞻性声明
本新闻稿中我们所做的声明可能包括并非历史事实的陈述,这些陈述根据经修订的《1933年证券法》(“证券法”)第27A条和经修订的《1934年证券交易法》(“交易法”)第21E条被视为前瞻性陈述。这些陈述可以通过诸如“目标”、“预期”、“相信”、“可能”、“估计”、“期望”、“预测”、“目的”、“打算”、“可能”、“计划”、“可能”、“潜在”、“寻求”、“将”以及这些词或类似表达的变化来识别,旨在识别前瞻性陈述。本新闻稿中的任何非历史事实的陈述,包括关于我们候选产品及其开发项目的临床和治疗潜力的陈述,包括icovamenib及icovamenib作为T1D和T2D治疗的潜力,以及我们对最佳剂量和目标患者群体的预期;我们的研究、开发和监管计划;我们候选产品及其开发项目的作用机制;我们正在进行和即将进行的临床试验的进展和启动,包括我们的食物效应研究(COVALENT-121)、我们针对严重胰岛素缺乏型2型糖尿病患者的IIb期试验(COVALENT-211)预计于2025年第四季度启动,以及与GLP-1疗法联合的II期试验(COVALENT-212)在2型糖尿病患者中预计于2025年第四季度启动;我们临床试验数据的预期可用性;我们与监管机构的计划互动及其时间安排,均可能被视为前瞻性陈述。我们希望这些前瞻性陈述受到证券法第27A条和交易法第21E条中包含的前瞻性陈述安全港条款的保护,并为此目的作出本声明以符合这些安全港条款。本新闻稿中的任何前瞻性陈述仅基于我们截至本发布日期的当前预期、估计和预测,并受许多可能导致实际结果与这些前瞻性陈述中所述或暗示的结果存在重大不利差异的风险和不确定性的影响,包括临床前研究或临床试验的初步或中期结果可能无法预测未来或最终结果,以及我们在临床前或临床开发中可能遇到延迟、患者入组延迟,以及我们正在进行和计划的临床试验及其他研发活动的启动、实施和完成的延迟风险。有关Biomea Fusion业务和运营的这些风险的详细描述包含在其向美国证券交易委员会("SEC")提交的定期文件中,包括其最近向SEC提交的定期报告及之后的后续文件。除非法律要求,Biomea Fusion明确拒绝更新任何前瞻性陈述的义务。抄送$诺和诺德(NVO)$ 诺和诺德,礼来。
Contact:
Meichiel Jennifer Weiss
Sr. Director of Investor Relations and Corporate Development
ir@biomeafusion.com
联系:
Meichiel Jennifer Weiss
投资者关系与企业发展高级董事
ir@biomeafusion.com
译文内容由第三方软件翻译。
以上内容仅用作资讯或教育之目的,不构成与富途相关的任何投资建议。富途竭力但不能保证上述全部内容的真实性、准确性和原创性。