康 · 学术 | Reaction of the Day No. 1564
(来源:康龙化成)
转自:康龙化成
One-Pot Bioisosteric Replacement of Alkyl Carboxylic Acids via Organic Photoredox Catalysis
Brittney A. Haney, Morgan T. Merriman, Siran Qian and David A. Nicewicz*
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599-3290, United States
—Org. Lett. 2025,doi: 10.1021/acs.orglett.5c04049
Recommended by Depei Meng_MC4

KEY WORDS: decarboxylation, photo chemistry, organic photoredox catalysis(反应类型), C(sp3)–C(sp2) (成键类型), carboxylic acids (原料), tetrazoles(产物), bioisosteres(其他)
ABSTRACT: Drug development is often hindered by the pharmacokinetic and physicochemical limitations of lead compounds. Bioisosteric replacement is commonly employed to bolster drug viability; however, methods for accessing drug analogs are often synthetically inefficient. To address this challenge, we disclose strategies to access carboxylic acid bioisosteres via organic photoredox catalysis. A one-pot method enables the direct conversion of carboxylic acids to their most common bioisostere, tetrazoles. Additional functionalization to an amidoxime intermediate affords access to three other bioisosteres: oxathiadiazolones, oxadiazolones, and oxadiazole thiones. HPLC lipophilicity measurements of carboxylic acids and bioisostere derivatives illustrate the synthetic value of these methods for improving lead compound viability.


(A)Tetrazoles as bioisosteres of carboxylic acids. (B )Precedented strategies for acid to subsequent bioisosteres. (C) Decarboxylative cyanation of aliphatic carboxylic acids to radiolabeled nitriles. (D) One-pot synthesis of tetraoles via a decarboxylative cyanation.
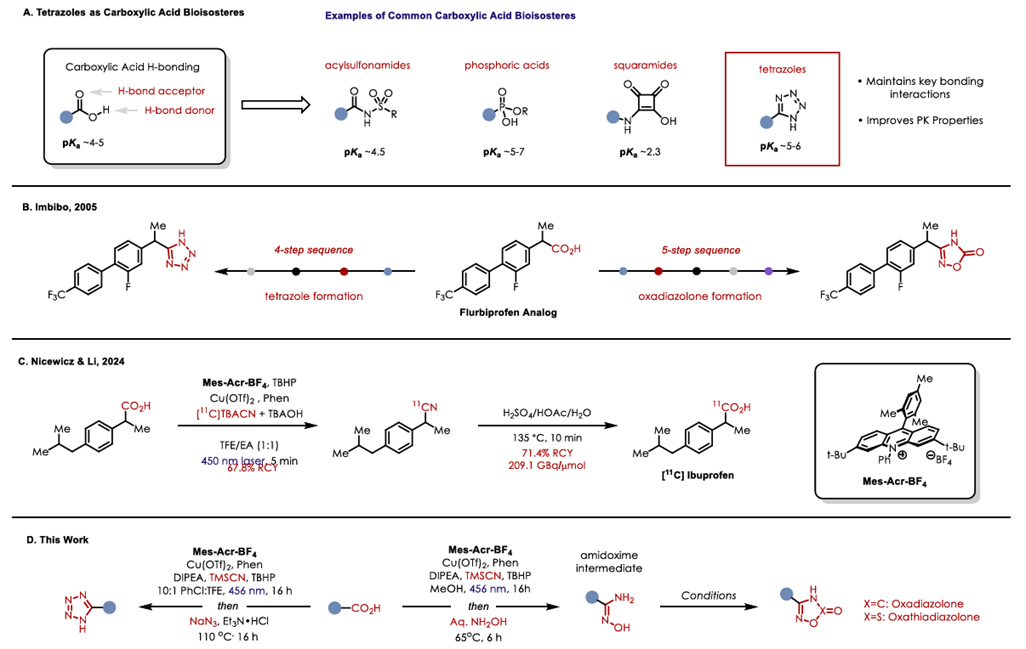

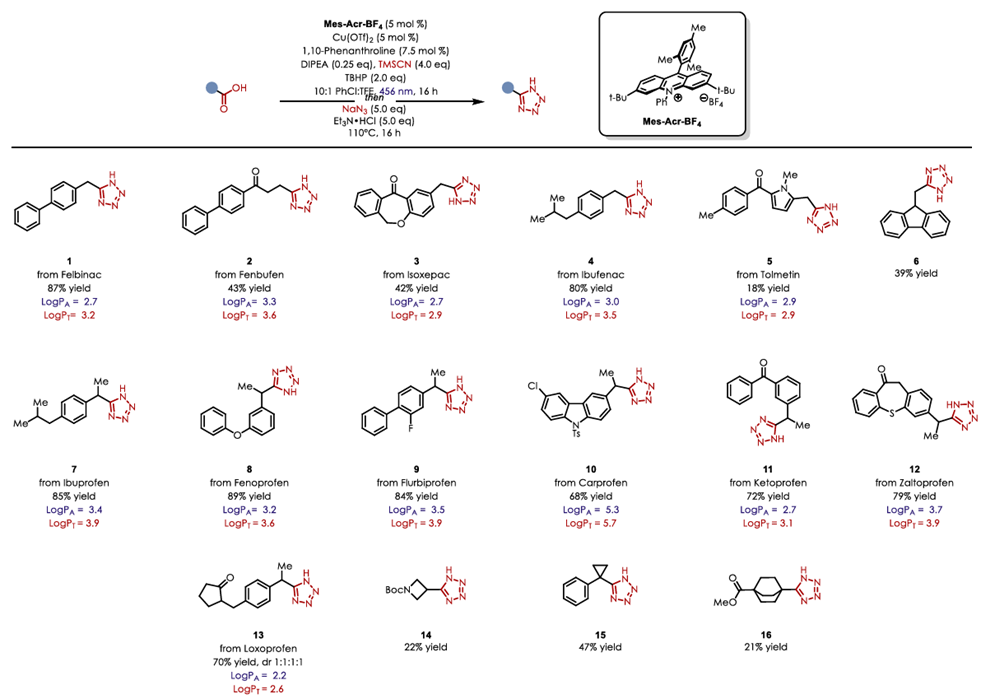

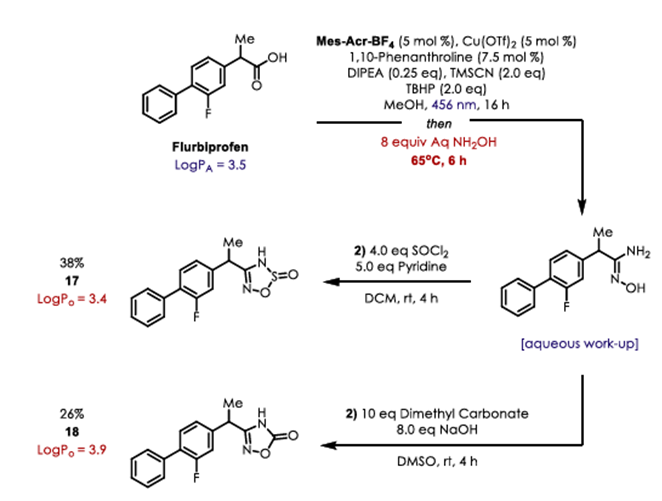
Synthetic routes to alternative bioisostere analogs for NSAIDs Flurbiprofen
In conclusion, Prof. Nicewicz grouphave demonstrated the use of organic photoredox catalysis for the direct conversion of carboxylic acidsto the correspondingtetrazoles and other pertinent bioisosteres. The one-pot conversion of carboxylic acids to the corresponding tetrazoles was successfully applied to a variety of drug molecules possessing a range of functionality in good yields by using mild conditions. More efficient routes toward oxathiadiazolone and oxadiazolone bioisosteres were also disclosed. From one established hit, three analogs with distinct PK and physicochemicalproperties can be synthesized, ideally providing biomedical researchers with additional tools for drug candidate development.
总地来说,Nicewicz教授团队成功利用有机光氧化还原催化实现了将羧酸直接转化为相应四唑化合物及其他相关生物电子等排体。该一锅法转化反应条件温和,可用于多种具有不同官能团的药物分子,并以良好收率获得目标四唑产物。研究还展示了通过该方法高效合成氧硫二氮唑酮与氧二氮唑酮生物等电子等排体。基于一个已验证的先导化合物,可同步构建三个具有不同药代动力学特性和理化性质的类似物,有望为生物医学研究者的候选药物开发提供更丰富的工具集。